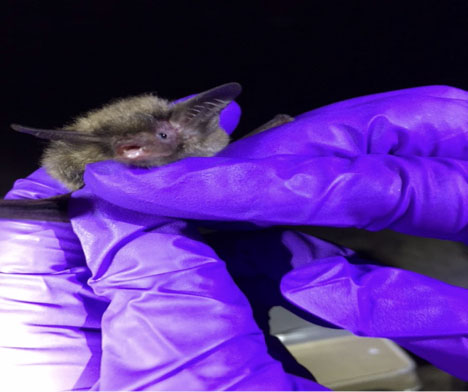
I grew up in Taiwan, which is a small island in southeastern Asia. In terms of the size, Taiwan is only two-thirds of New York State, and half of it is covered by mountains. Thus, Taiwan overall is so steep that it is almost impossible to find somewhere flat; therefore, I’ve been eager to see flatness. On the other hand, even though the biodiversity is generally higher, there are not many large mammals in Taiwan due to the limited area. Mammals, moreover, tend to hide in woods when humans are approaching. Seeing a mammal during fieldwork is a huge surprise for researchers in Taiwan.
My two thirsts were fulfilled simultaneously by Etosha National Park, the size of which is two-thirds of Taiwan. There are remarkably flat plains with several seeable mammals on them. I sometimes went to the waterhole by Okaukuejo camp. It was amazing to see herbivores coming to the waterhole endlessly. Mammals supported by that one waterhole—I guess—are more than mammals supported by a river in Taiwan!
One of my jobs on this visit to Etosha was placing cameras to monitor animal reactions toward carcasses/carcass sites, in order to evaluate the possible risk being infected by anthrax. I personally summarized a few important points for you to keep in mind when putting up cameras at new carcass sites. First of all, before getting out of a car, it is required to check whether predators are around. This is in fact not only for fresh carcasses but also for every time getting out of a car in the field. Second, if there are predators around, you should estimate how far they are from the carcass site and how long it would take for them to reach the site. Once safety is confirmed, you can get out of the car and place the cameras. However, it is important to let at least one person keep watch on any predators and, moreover, to leave doors open in case quickly retreating is needed. Last but not least, when getting close to fresh carcasses, you should hold your breath if you are not familiar with working with carcasses. Otherwise, it will be like enjoying a famous food in Taiwan—stinky tofu.
After putting up a few cameras, I chatted with Wendy’s husband, Yeti.
Me: “If lions were attacking us during our fieldwork, can I say I saw lions hunting?”
Yeti: “But before that, first, you need to survive.”